
2
octane. The bottle is closed immediately (gas-tight) and
placed in a water bath at 80 °C for 1 h with intermittent
shaking and inverting the bottle every 20 min. After
cooling the bottle to < 20 ˚C by ice water, a 1-2 mL
aliquot of the upper isooctane layer is transferred into
a micro centrifuge tube, and centrifuged at 5000 rpm
for 5 min at 10 °C. The supernatant is then transferred
into GC vials, and the residues of DTCs are estimated
by determining the CS
2
concentration by GC-MS. The
sample preparation procedure depending on the type of
food used takes approx. 1-2 hrs.
Preparation of Standard Solutions and
Reaction Mixture
For method validation, Thiram (99.5% purity) was used
as representative DTC compound considering its simple
structure (1 mole of Thiram = 2 mole of CS
2
).
Carbon disulphide standard solution
A stock solution of CS
2
(2000 µg/mL) was prepared by
accurately pipetting out 79.0 µL of CS
2
into a volumetric
flask (certified A class, 50 mL) containing approximately
45 mL of iso-octane and made up to 50 mL with iso-
octane. The CS
2
stock solution was kept in a refrigerator
at -20 °C and used within two days of preparation. The
CS
2
working standard solutions of 200 and 20 µg/mL
concentrations (10 mL each) were prepared by serial
dilution of stock solution with iso-octane.
Standard Solution of Thiram
10 mg (± 0.05) of Thiram was weighed into a 10 mL
volumetric flask (certified A class) and dissolved in ethyl
acetate up to the mark to get a stock solution of 1000
µg/mL. A 100 µg/mL Thiram working standard was
prepared from stock solution by 10-times dilution.
Preparation of Reaction Mixture
An amount of 30 g of tin (II) chloride was accurately
weighed in the 1000 mL volumetric flask (certified A
class) to which 1000 mL of concentrated HCL (35%)
was added. The solution was then gradually added to
1000 mL water with continuous stirring until a clear
solution was obtained.
Calibration Standards
Calibration standard solutions of CS
2
at six different
concentration levels (0.04, 0.08, 0.16, 0.32, 0.64, and 1.3
µg/mL) were prepared by appropriate dilutions of 20 µg/
mL CS
2
working standard in iso-octane.
Matrix matched standards at the same concentrations
were prepared by spiking the iso-octane extract of fresh
control grapes, potato, tomato, green chili, and eggplant
(all organically grown) using the following formula
derived from above conversion of Thiram to CS
2
:
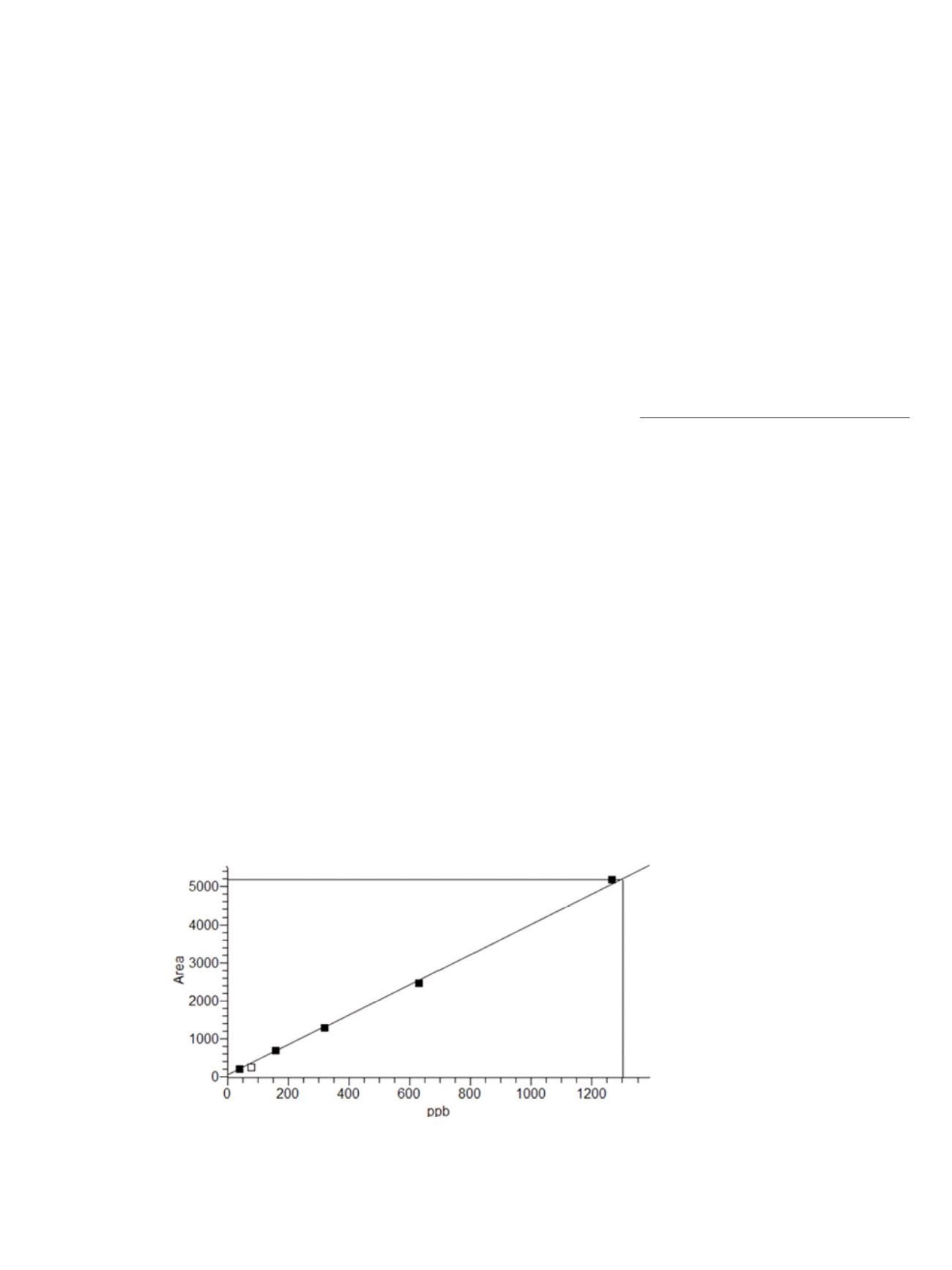
Figure 2. Calibration curve, range 0.04 - 1.300 µg/mL Thiram matrix spike, R
2
= 0.9990.
Before the preparation of matrix matched standards, the
control samples were carefully monitored for absence of
DTCs (in terms of CS
2
).
Experimental Conditions
A Thermo Scientific™ TRACE GC Ultra™ gas
chromatograph equipped with Thermo Scientific™
Triplus™ RSH liquid autosampler and coupled to
a Thermo Scientific™ ITQ™ 900 ion trap mass
spectrometer was used for analysis. See Tables 1 and 2
for instrument parameters.
Two GC columns of different polarity, stationary phase,
and film thickness have been evaluated. The first column
was a medium polarity cyanopropylphenyl phase (6%
cyanopropylphenyl/94% dimethyl polysiloxane, 30
m x 0.32 mm ID, 1.8 µm film thickness, e.g. Thermo
Scientific™ TraceGOLD™ TG-624, p/n 26085-3390) and
as a second column a low polarity 5%-phenyl stationary
Spike quantity=
Concentration to be achive*weight of the sample
0.6333*concentration of the stock solution



















