
Chromatographic Conditions
Instruments:
Thermo Scientific Accela pump
Thermo Scientific Accela Autosampler
Columns:
Hypersil GOLD PFP, 1.9 µm, 100 x 2.1 mm
Flow Rate:
0.5 mL/min
Mobile Phase:
A: water, 1 mM ammonium formate
B: methanol
Gradients:
Time (min)
A(%)
B(%)
µL/min
0.0
80.0
20.0
500
10.0
45.0
55.0
500
12.0
20.0
80.0
500
12.1
5.0
95.0
500
12.9
5.0
95.0
500
13.0
80.0
20.0
500
15.0
80.0
20.0
500
Injection Volume: 2 µL partial loop injection, 25 µL loop size
Mass Spectrometer Conditions
Instrument:
MSQ Plus Mass Detector
Ionization:
Atmospheric Pressure Chemical Ionization (APCI)
Polarity:
Positive and Negative
Probe Temperature: 350 °C
Cone Voltage:
60.0 V
Scan Mode:
Full scan with mass range of 50-400 amu
or selected ion monitoring (SIM)
Corona Current:
30 µA
Scan Time:
0.5 s for full scan, 0.25 s for SIM
Results and Discussion
UHPLC Separation and MS Detection
USEPA 8330 method provides sensitive UV detection for
nitroaromatic and nitroamine explosives. However, two
analytical columns with different stationary phases are
required to separate and identify the isomers, 2,4-DNT
and 2,6-DNT, 4-A-2,6-DNT and 2-A-4,6-DNT, which
make this method time consuming and results in low
sample throughput.
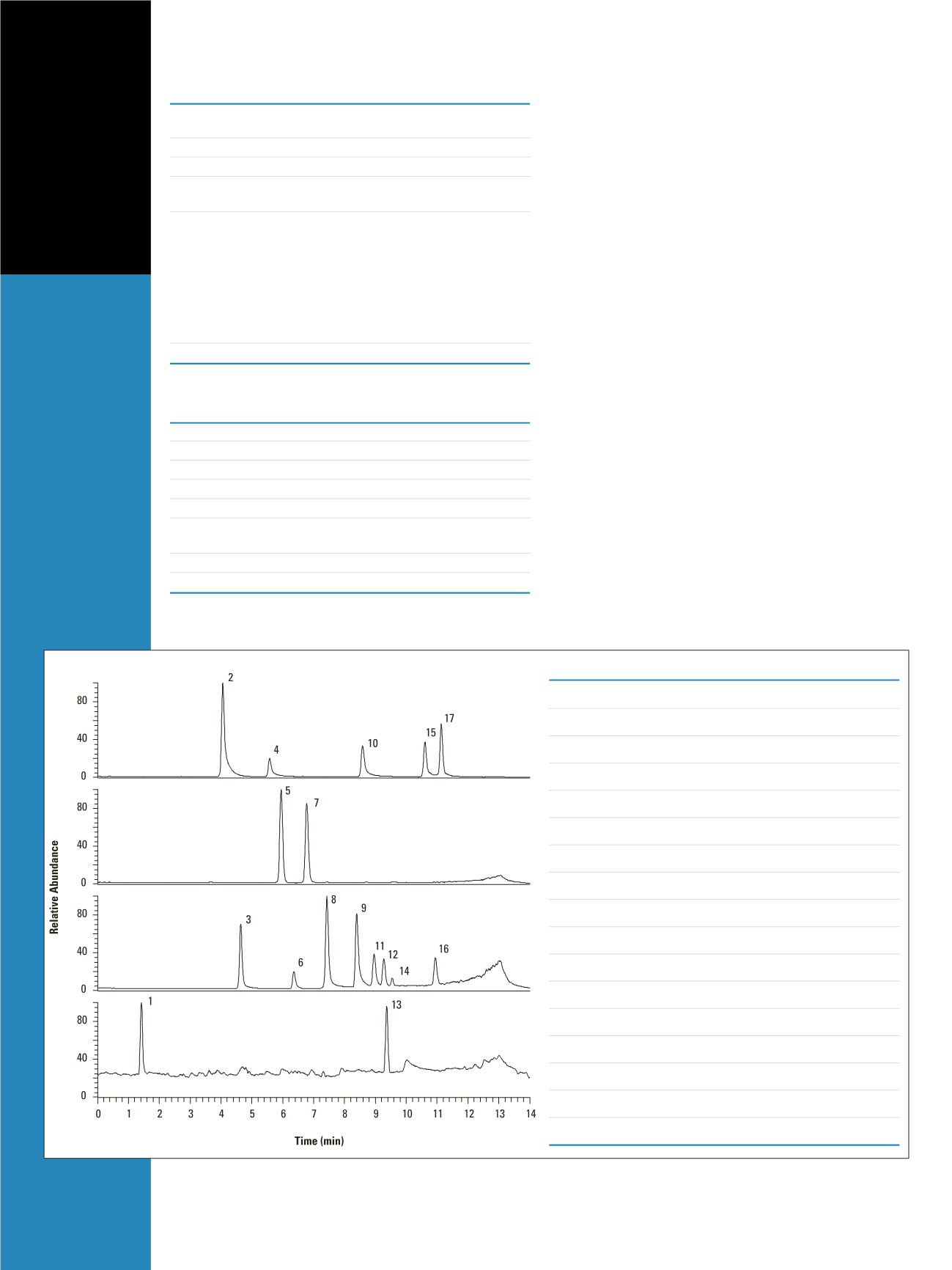
The simultaneous separation and detection of
seventeen explosive compounds was achieved through
UHPLC/MS, using the Thermo Scientific Accela system
with a fast scanning, single quadrupole mass spectrometer
(Figure 1). Water and methanol were used as the mobile
phases and the optimized gradient is shown in the
Chromatographic Conditions. The elution order of the
compounds and their retention times are shown in Figure 1.
Hypersil GOLD
™
PFP has a fluorinated phenyl group in
the stationary phase which improves selectivity towards
aromatic compounds. It also provides better resolutions
for polar compounds containing hydroxyl, carboxyl, nitro
or other polar groups. Eight nitroaromatic compounds,
two nitroamine compounds, five nitrate ester compounds
and two peroxides were separated with baseline resolution
on a Hypersil GOLD PFP, 1.9 µm, 100 x 2.1 mm column.
The isomer pairs, 2,4-DNT and 2,6-DNT , 4-A-2,6-DNT
and 2-A-4,6-DNT, were separated with the peak resolution
of 2.8 and 7.3 respectively (Peaks 9 and 11, 12 and 16).
Figure 1: UHPLC/MS separation and detection of the 17 explosives standard with negative APCI (a-c) and positive APCI (d) ionizations. a) Extracted ion
chromatogram at
m/z
of 61.96; b) Extracted ion chromatogram at
m/z
of 102.05; c) Extracted ion chromatogram at
m/z
of 213.02, 168.09, 227.01, 182.07,
197.04 and 241.02; d) Extracted ion chromatogram at
m/z
of 209.04 and 348.08.
Peak
Compound
Retention Time (min)
1
HMTD
1.42
2
EGDN
4.06
3
TNB
4.64
4
DEGDN
5.58
5
HMX
5.95
6
1,3-DNB
6.36
7
RDX
6.77
8
TNT
7.43
9
2,6-DNT
8.40
10
NG
8.58
11
2,4-DNT
8.96
12
4-A-2,6-DNT
9.28
13
TATP
9.37
14
TETRYL
9.55
15
TMETN
10.60
16
2-A-4,6-DNT
10.94
17
PETN
11.13
Page 2 of 8
d
c
b
a



















