4
The base esterification process resulted in fewer FAME peaks observed in the fat samples compared
to the acid esterification process (see Table 2 for comparison). Some emulsification occurred during
phase separation and the esterified solution required filtering with a syringe filter prior to GC
analysis.
In contrast, acidic esterification produced more FAME peaks than base esterification. The strong
Lewis acid BF
3
-methanol can more efficiently esterify fatty acids compared with the base
esterification method, with no white emulsion appearing when reaction is worked up with water.
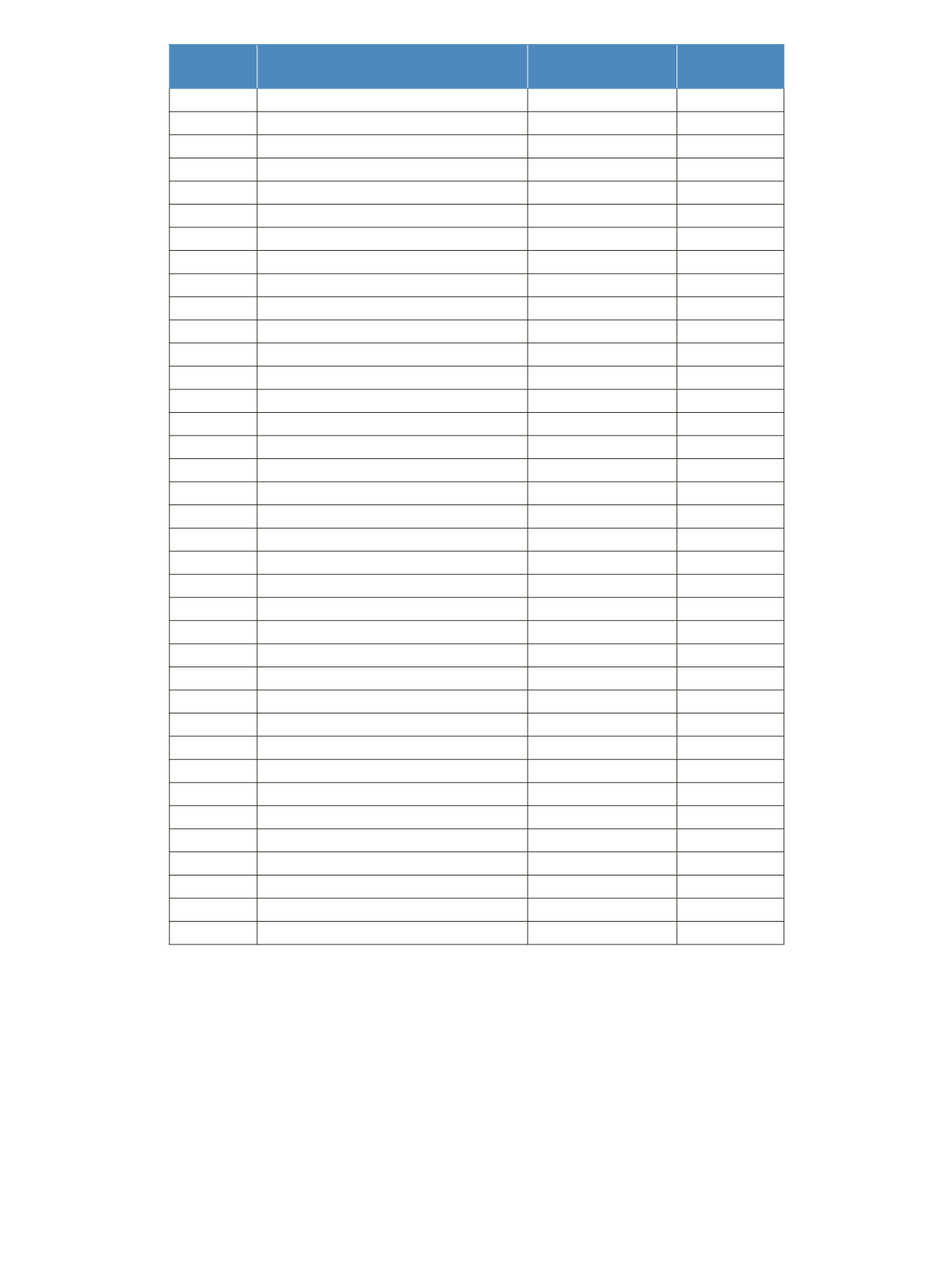
Elution order
Compound
Concentration %wt/wt
t
R
/min
1
Methyl butyrate (C4:0)
4
13.40
2
Methyl caproate (C6:0)
4
15.53
3
Methyl capylate (C8:0)
4
19.60
4
Methyl decanoate (C10:0)
4
25.90
5
Methyl undecanoate (C11:0)
2
29.56
6
Methyl dodecanoate (C12:0)
4
33.50
7
Methyl tridecanoate (C13:0)
2
37.36
8
Methyl myristate (C14:0)
4
41.30
9
Methyl myristoleate (C14:1 [
cis
-9])
2
43.36
10
Methyl pentadecanoate (C15:0)
2
45.01
11
Methyl pentadenoate (C15:1 [
cis
-10])
2
47.12
12
Methyl palmitate (C16:0)
6
48.79
13
Methyl palmitoleate (C16:1 [
cis
-9])
2
50.30
14
Methyl heptadecanoate (C17:0)
2
52.18
15
Methyl heptadenoate (C17:1 [
cis
-10])
2
53.78
16
Methyl stearate (C18:0)
4
55.67
17
Methyl octadecenoate (C18:1 [
trans
-9])
2
56.38
18
Methyl oleate (C18:1 [
cis
-9])
4
56.96
19
Methyl linoleaidate(C18:2 [
trans
-9,12])
2
57.79
20
Methyl linoleate (C18:2 [
cis
-9,12])
2
59.06
21
Methyl arachidate (C20:0)
4
60.48
22
Methyl linolenate (C18:3 [
cis
-6,9,12])
2
61.61
23
Methyl (C20:1 [
cis
-11])
2
62.01
24
Methyl linolenate (C18:3 [
cis
-9,12,15])
2
63.20
25
Methyl heneicosanoate(C21:0)
2
64.96
26
Methyl eicosadienoate (C20:2 [
cis
-11,14])
2
65.31
27
Methyl behenate (C22:0 FAME)
4
66.66
28
Methyl eicosatrienoate (C20:3 [
cis
-8,11,14)
2
67.60
29
Methyl erucate (C22:1 [
cis
-13])
2
67.72
30
Methyl eicosatrienoate (C20:3 [
cis
-11,14,17])
2
67.87
31
Methyl arachidonate (C20:4 [
cis
-5,8,77,14])
2
69.06
32
Methyl tricosanoate (C23:0)
2
70.06
33
Methyl docosadienoate (C22:2 [
cis
-13,16])
2
70.57
34
Methyl lignocerate (C24:0)
4
71.06
35
Methyl
cis
-5,8,11,14,17-eicosapentaenoate
2
73.41
36
Methyl nervonate (C24:1 [
cis
-15])
2
74.74
37
Methyl
cis
-4,7,10,13,16-docosahexenoate
2
77.23
Table 1: FAMEs according to the elution order and retention times for the reference standard


