
Identification and Quantitation of
Microcystins by Targeted Full-Scan
LC-MS/MS
Terry Zhang, Reiko Kiyonami, Leo Wang and Guifeng Jiang
Thermo Fisher Scientific, San Jose, CA, USA
Application Note 569
Key Words
Velos Pro, UltiMate, Water Analysis, Cyanobacteria, Microcystin
Goal
Develop a simple and sensitive LC-MS method for definitive identification
and quantitation of microcystins in water.
Introduction
Cyanobacteria, commonly referred to as blue-green algae,
are photosynthetic prokaryotes that occur naturally in
surface waters. They contribute significantly to primary
production and nutrient cycling. Eutrophic, warm and
low turbulent conditions in freshwater bodies typically
promote the dominance of cyanobacteria within
phytoplankton communities. Excessive proliferation of
cyanobacteria leads to blooms that disrupt ecosystems,
adversely affect the taste and odor of water, and increase
water treatment costs. Blooms of toxic cyanobacteria
species in surface drinking water sources and recreational
waters threaten human health. Gastrointestinal illness,
skin irritation, and death following renal dialysis have
been attributed to acute cyanotoxin exposure. Chronic
exposure can cause liver damage and may be associated
with primary liver cancer.
1
The incidence and severity of
cyanobacterial blooms are increasing globally, underscoring
the importance of cyanotoxin monitoring.
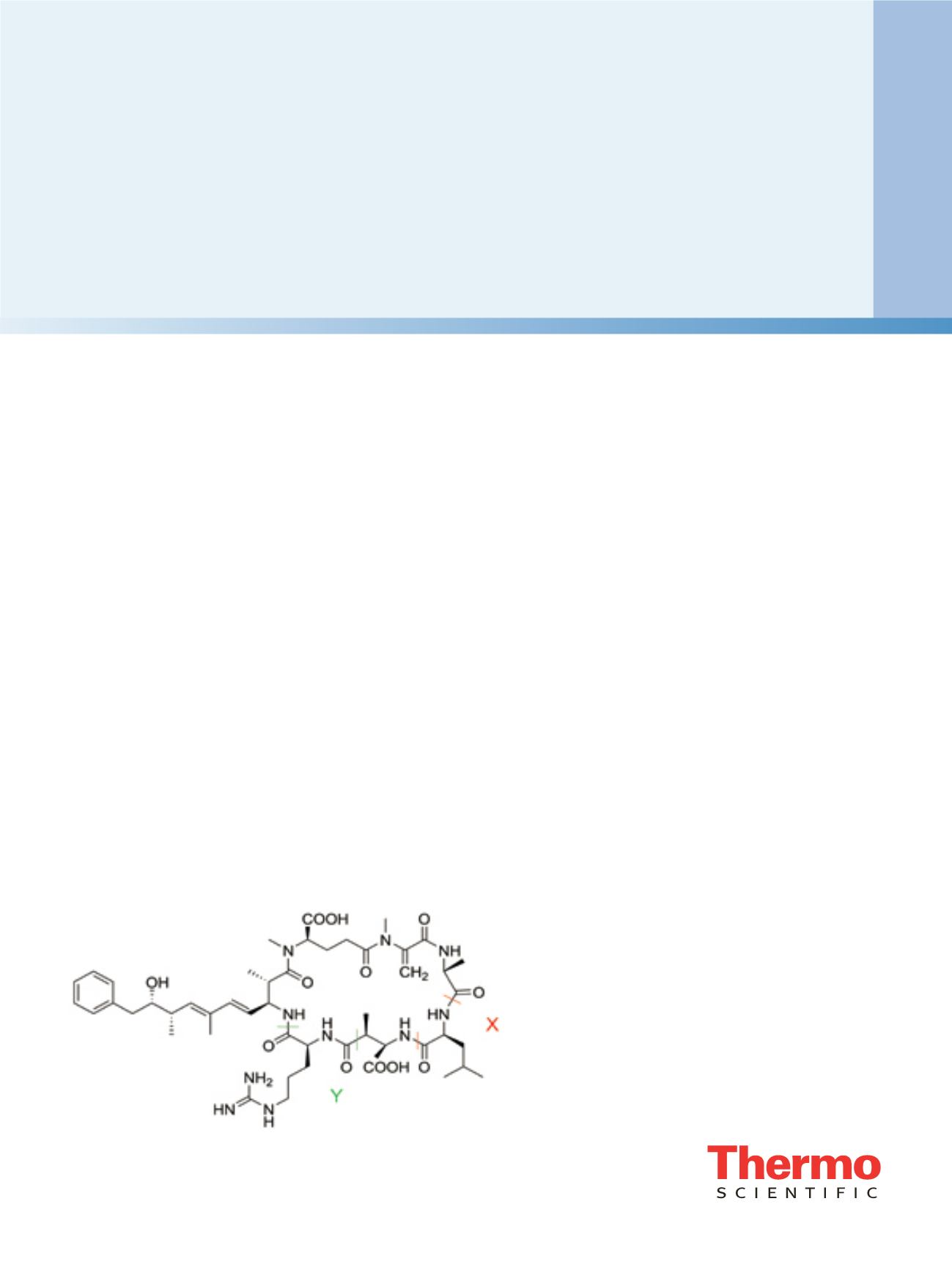
Figure 1. The chemical structure of MC-LR contains leucine (L)
and arginine (R) at positions X and Y, respectively. Microcystin
nomenclature is based on the L-amino acids present at these two
positions.
The most commonly encountered cyanotoxins are the
microcystins, a group of hepatotoxic cyclic heptapeptides
produced by various genera of cyanobacteria, including
Microcystis, Planktothrix, and Anabaena. The chemical
structure of a microcystin, depicted in Figure 1, is
characterized by the presence of the amino acid 3-amino-
9-methoxy-2,6,8-trimethyl-10-phenyl-deca-4,6-dienoic
acid (Adda), which modulates the biological activity of
these toxins, and N-methyldehydroalanine (Mdha).
Microcystin nomenclature is based on the L-amino acids
present at two positions (X and Y in Figure 1) in the
molecule. Over 80 structural variants are known,
differentiated by the two variable L-amino acids as well as
by chain modifications. The inhibition of serine/threonine
protein phosphatases type 1 and 2A is considered the
major mechanism of microcystin toxicity. Microcystin-LR,
one of the most prevalent and potent microcystins, is
designated as possibly carcinogenic to humans by the
International Agency for Research on Cancer (IARC).
2
The potential risk of chronic exposure to microcystins in
drinking water supplies prompted the World Health
Organization (WHO) to issue a provisional guideline of
1 μg/L as the maximum concentration of total microcystin-LR
(free plus cell-bound) in drinking water.
3
Many national
and regional governments have since adopted this
guideline value directly or have established slightly
modified variants.



















