
Part of Thermo Fisher Scientific
WWW THERMOSCIENTIFIC COM
Legal Notices: ©2011 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries.
This information is presented as an example of the capabilities of Thermo Fisher Scientific Inc. products. It is not intended to encourage use of these products in
any manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change. Not all products are available
in all countries. Please consult your local sales representative for details.
Thermo Fisher Scientific,
San Jose, CA USA is ISO Certified.
In addition to these
offices, Thermo Fisher
Scientific maintains
a network of represen-
tative organizations
throughout the world.
!FRICA /THER
+27 11 570 1840
Australia
+61 3 9757 4300
Austria
+43 1 333 50 34 0
Belgium
+32 53 73 42 41
Canada
+1 800 530 8447
#HINA
+86 10 8419 3588
Denmark
+45 70 23 62 60
%UROPE /THER
+43 1 333 50 34 0
&INLAND .ORWAY
Sweden
+46 8 556 468 00
&RANCE
+33 1 60 92 48 00
Germany
+49 6103 408 1014
India
+91 22 6742 9434
Italy
+39 02 950 591
Japan
+81 45 453 9100
Latin America
+1 561 688 8700
Middle East
+43 1 333 50 34 0
.ETHERLANDS
+31 76 579 55 55
New Zealand
+64 9 980 6700
2USSIA #)3
+43 1 333 50 34 0
3OUTH !FRICA
+27 11 570 1840
Spain
+34 914 845 965
Switzerland
+41 61 716 77 00
UK
+44 1442 233555
USA
+1 800 532 4752
AN63401_E 04/11S
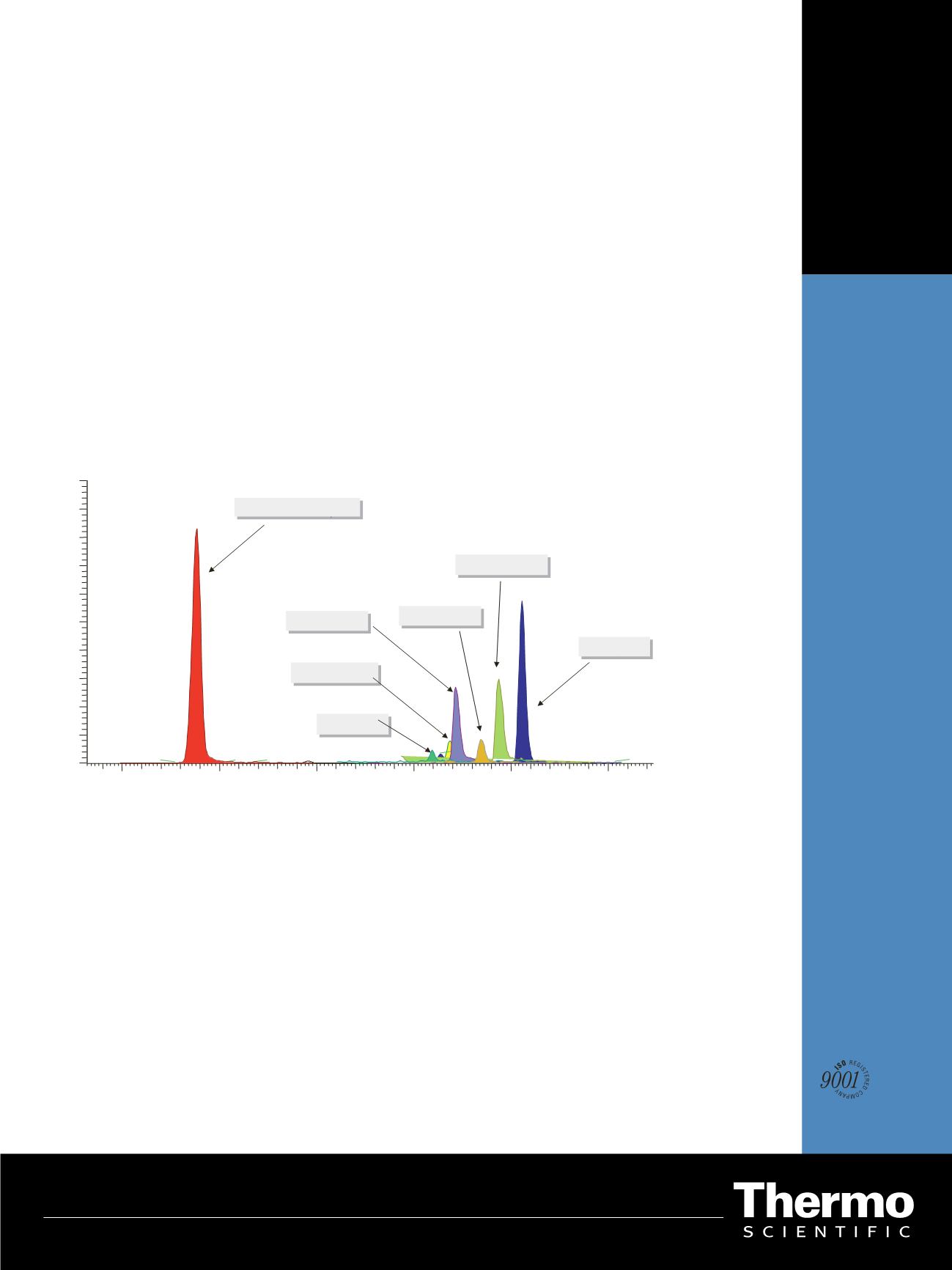
Figure 2
: chromatogram obtained from a real sample
acquired using the Timed-SRM LC/MSMS
2.0
2.5
3.0
3.5
4.0
4.5
Time (min)
0
10
20
30
40
50
60
70
80
90
100
Relative Abundance
2.39
4.06
3.94
3.72
3.84
Oxazepam
Clonazepam
Nordazepam
Temazepam
Lormetazepam
Diazepam
7 Amino-clonazepam
3.94
3.46
3.69
Figure 1. Chromatogram obtained from a real sample acquired using the T-SRM UHPLC-MS/MS method
Inter-method variability was determined by processing
five replicates of four calibration levels in four different
batches run on four different days. All values were below
15% and therefore within the guidelines set for a validated
LC-MS/MS method.
Extraction efficiency also was evaluated and calculated
at three concentration levels: 10 ng/mL, 100 ng/mL and
300 ng/mL. Values were between 50% and 100%, except
for 7 amino-clonazepam which was around 30%.
The lower limit of quantitation (LLOQ) and the limit
of detection (LOD) of the compounds were determined
based on the calibration curve of S/N ratio versus concen-
tration and the definitions of LOQ and LOD using
S/N = 10 and 3. LLOQs were between 0.1 and 3 ng/mL
for all molecules. Figure 1 shows the chromatogram
obtained from a real sample acquired using the developed
UHPLC-MS/MS method.
Conclusion
A rapid UHPLC-MS/MS method for quantifying
benzodiazepines in whole blood samples was developed
for forensic toxicology. The precision of the analysis meets
current consensus guidelines. A T-SRM method was used
to increase the acquisition time per compound and achieve
better signal-to-noise ratios for the analytes.



















