
5
Thermo Scienti c Poster Note
•
PN63784_E 03/13S
rcept
R
2
00483
42.9
0.9935
0.48
04033
81.7
0.9967
0.26
2222
0.8
0.9913
0.46
09083
37.8
0.9980
0.10
04005
64
0.9964
0.22
2377
1.8
0.9911
1.44
20596
4.0
0.9894
0.69
0121
83.9
0.9919
0.46
02118
63.0
0.9943
0.32
Concentration
BORT
DASA
SUNI
Accuracy
Precision
Accuracy
Precision
Accuracy
Precision
2
98.4
19.8
106.9
16.2
119.8
20.0
5
93.2
19.5
101.5
8.2
99.7
6.3
10
93.9
8.9
98.3
11.9
97.6
7.0
20
108.2
13.1
97.8
6.0
93.9
11.0
50
104.0
10.1
97.2
7.7
90.7
5.1
100
98.2
5.8
98.4
5.6
91.3
4.0
250
99.5
3.9
102.2
3.0
105.8
2.1
Concentration
ERLO
IMAT
LAPA
Accuracy
Precision
Accuracy
Precision
Accuracy
Precision
50
91.8
13.8
93.4
12.8
105.5
7.7
100
94.3
10.5
98.1
9.9
96.8
6.6
200
113.9
7.1
107.6
7.8
109.2
6.1
500
109.0
5.7
98.3
5.9
90.5
7.8
1000
103.5
5.1
100.2
5.1
96.8
6.1
2000
101.4
4.4
99.2
3.8
99.6
4.8
3500
94.9
3.6
100.8
2.3
101.9
2.7
Concentration
NILO
SORA
VAND
Accuracy
Precision
Accuracy
Precision
Accuracy
Precision
50
111.5
7.8
113.1
5.4
86.5
16.2
100
99.7
4.6
98.9
3.3
91.7
11.0
200
111.1
4.5
108.2
5.8
111.2
17.4
500
98.5
5.3
91.8
5.7
103.8
10.7
1000
93.0
7.0
91.2
8.4
108.5
5.0
2000
97.5
3.3
98.3
2.7
101.3
2.4
3500
103.2
3.0
105.3
3.3
96.0
3.3
d curves, prepared from different
sma on twenty consecutive days.
s the regression coefficients were
pted for sorafenib (mean r
2
0.9894)
eorical values, mean and standard
nominal concentration used in the
uracies were calculated for each
s were ranging between 0.9987 to
different from 1 (p<0.0001). The
A and SUNI and 50 ng/mL for the
mple containing each TKI.
Accuracy and precision
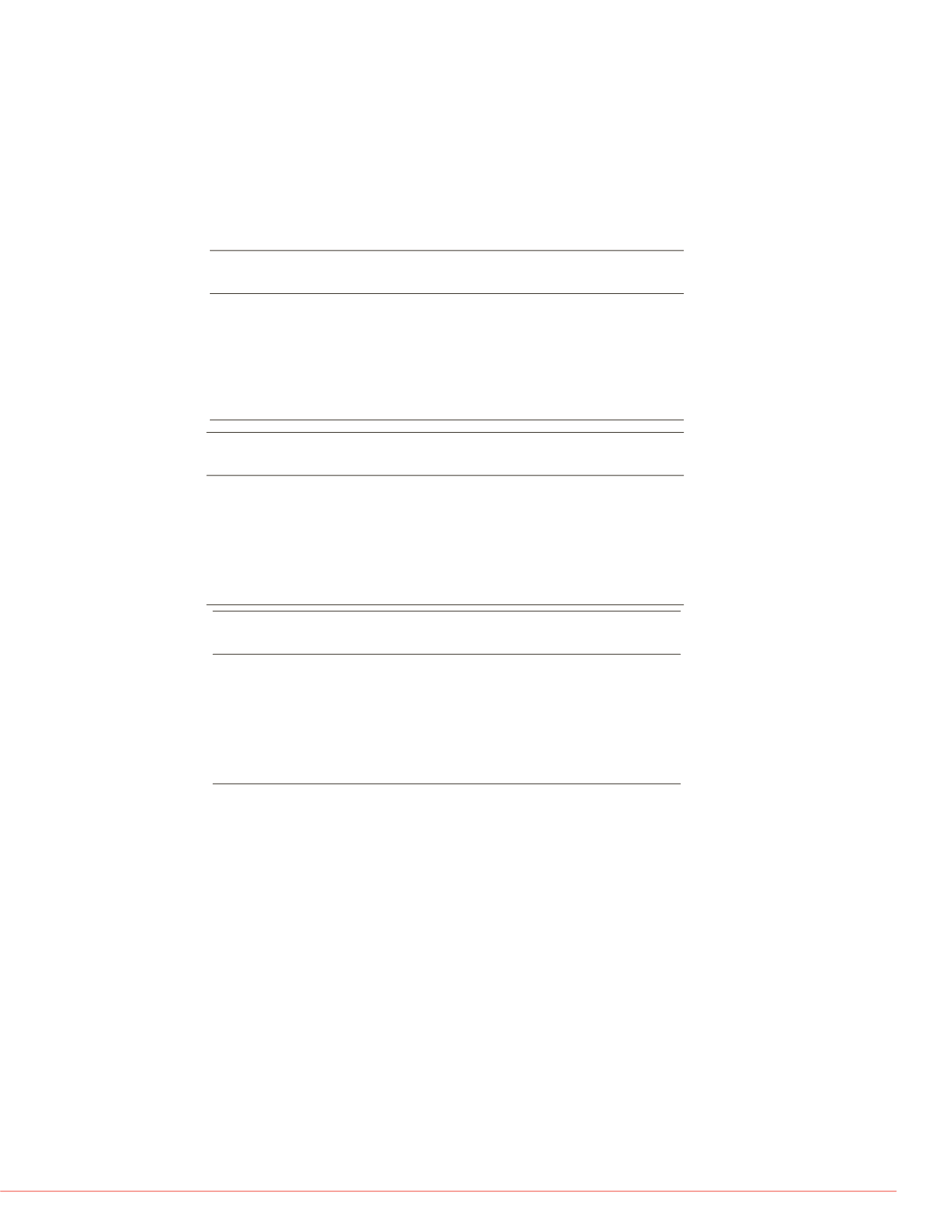
Precision and accuracy determined with 3 and 4 controls samples are given in Table
3. The levels of control samples were selected to reflect low, medium and high range
of the two sets of calibration curves. They were chosen to encompass the clinically
range of concentrations found in patients plasma. The mean intra-assay precision was
similar over the entire concentration range and lower than 8.2 %. Overall, the mean
inter-day precision was good with CVs within 5.3 and 13.8%. The intra-assay and
inter-assay
bias from the nominal concentrations of QCs for each considered TKI
were contained between and 86.8 and 113.5 %. Ratios of ion transitions were
reproducible for all TKIs and standard deviation for all of them below 25%.
fficient correlations (
r
2
) for 9TKIs
Extraction recovery and matrix ef
The assessment of matrix effects and extracti
value above or below 100% for the ma
enhancement or suppression, respectively. Ma
ranged from 84.6 to 109 % and 84.0 to 10
were ranged from 77.8 to 93.3 % for lowe
medium concentrations and from 79.8 to 10
extraction recovery of D8-imatinib was
hyperbilirubinemia, hyperlipemia and haemol
medium CQs.
Stability
The stability of TKIs in human plasma sampl
samples left at room temperature up to 48h.
15% of starting concentrations indicating that
excepted for lapatinib which decreases of -36
48h. It has been demonstrated that lapatinib
is sensitive to light and decreases by -15%
contrast, all TKIs in plasma samples left duri
were found stable.
QC samples prepared in human plasma u
showed no significant degradation (variation <
Long-term stability studies indicated that all a
when stored at -70
°
C for 150 days (ratios bet
7.9%).
The stability of stock solutions held at -70
°
showed decrease less than 6% for each analy
In neutral extracts, all analytes were stable u
without any degradation allowing more t
simultaneously within a single chromatographi
External quality controls
The external quality controls (low and hi
laboratories), nilotinib and dasatinib (9 laborat
to 101.4%) in comparison to data obtained fro
Application to biological samples
We applied the assay to the analysis of sam
imatinib, nilotinib, dasatinib, sunitinib or sorafe
DASA, IMAT and NILO were frequently dete
leukemia (n=75). In 71 patients treated with
detected though concentrations were aroun
Among these 71 patients, 45 % of them pr
associated with a trough concentration h
recommended [50].
We applied the assay to samples provided fr
mg sunitinib for a renal carcinoma. The profile
this obese woman showed no difference with
patients without obesity.
Conclusion
In overall, the method that has been develope
concerns nine inhibitors of tyrosine kinase ac
performed using confirmation/quantification io
simple and therefore used in a routine envir
possible to add new TKIs that could potentiall
and performed a partial analytical valida
concentrations allow to carry out some pharm
Table 3:
Assay performance data of the low calibration samples for BORT, DASA, SUNI and of the high
calibration samples for ERLO, IMAT, LAPA, NILO, SORA, VAND in human plasma (n=20)
Selectivity and specificity
No peaks from endogeneous compounds were observed at the drugs retention time
in any of the 10 blank plasma extracts evaluated. The endogeneous responses in
blank plasma were always below 6.5 % of the signal at the LLOQ of 2 ng/mL for
BORT, DASA, SUNI and at 50 ng/mL for the others. The endogeneous responses in
plasma provided from polymedicated patients were always less than 7.1% of the
signal at each LLOQ. There were no effects of others concomitant treatments (40
mg/l of amikacin, 20 mg/l of gentamycin, 25 mg/l of vancomycin, ceftazidime,
imipenem and cisplatin, 0.5 mg/l of morphine, 3 mg/l of docetaxel, 5 mg/l of
voriconazole, posaconazole, itraconazole and fluconazole).
All trademarks are the property of Thermo Fisher
This information is not intended to encourage use
infringe the intellectual property rights of others.



















